Choosing the Right Modeling Strategy: Explanatory, Exploratory, and Predictive Approaches in Clinical Research
Table: Modeling Strategies in Clinical Research Dimension Explanatory Model Exploratory Model Predictive Model Primary Purpose Test a...

Clinical Guide to the Screening and Management of Dyslipidemia (Thai Guidelines 2024)
Introduction Dyslipidemia remains a significant risk factor for cardiovascular disease (CVD), the leading cause of morbidity and...

Logistic Regression in Clinical Research: Theory, Application, and Interpretation
Introduction Logistic regression is one of the most frequently applied analytical tools in clinical epidemiology and health data science....

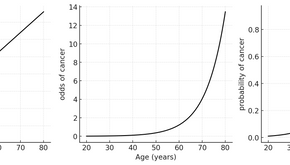
Understanding Odds and Odds Ratios in Clinical Epidemiology
Introduction In clinical epidemiology and biostatistics, the concept of "odds" frequently arises in the analysis of binary outcomes,...

IPTW (Inverse Probability of Treatment Weighting) Made Easy: How to Rebalance Observational Data Like a Randomized Trial
In the real world, doctors don’t flip a coin to assign treatments. So when we try to compare outcomes between treated and untreated...

From Chaos to Causal Clarity: Balancing Scores and Propensity Scores Explained Simply
Introduction: Why This Matters Imagine you're trying to compare two treatments, but your patients weren't randomized. You're worried that...

Three Tools to Estimate Causal Effects Without a Trial: Model-Based, Standardisation, and Matching
Imagine you're a clinician-researcher trying to answer a deceptively simple question: “Do apples help lower blood pressure?” You can’t...

Conditional vs. Marginal Odds Ratios: Why Your Logistic Regression May Be Lying to You
👩⚕️ The Situation You’re analyzing your study: Did Drug A reduce the risk of infection compared to Drug B? You run a logistic...

Causal Thinking in Observational Studies: Matching, Propensity Scores, and IPTW Explained
When we want to know if a treatment truly causes a better outcome—especially in observational studies—we need more than just statistics....


Outcome-Driven Regression: Choosing the Right Model Based on Y in Clinical Research
Why start with Y ? Because the distribution and scale of the outcome (not the exposure) dictate: The likelihood function behind the...

How to Order LPRC Transfusion (Also CPM) for Symptomatic Anemia in IPD Settings
In non-emergency IPD (inpatient department) settings, transfusion of Leukocyte-Poor Red Cells (LPRC) is commonly done for symptomatic...

What is Marginalisation in Clinical Statistics?
Marginalisation refers to the process of transforming effect estimates derived from specific subgroups (i.e., conditional on covariates...

Collapsibility Explained with Clinical Logic: When Crude Equals Adjusted (and When It Doesn’t)
🧩 Collapsible = “Crude ≈ Adjusted” If you don’t adjust for age/sex and then do adjust , the result doesn’t change much. 💊 Example:...

What Is Collapsibility in Clinical Statistics?
Collapsibility is a property of effect measures that determines whether the measure changes when you (statistically) adjust or don’t...

Choosing the Right Regression Model: A Visual Guide for Outcome Types in Clinical Research
In clinical epidemiology and biostatistics, choosing the correct regression model hinges on the characteristics of the outcome variable...

Logistic Regression สำหรับข้อมูล Binary: ทำไมใช้ได้ แม้ Y มีแค่ 0 กับ 1
🔍 ปัญหาเริ่มต้น: “ถ้า Y มีแค่ 1 หรือ 0 แล้วจะวิเคราะห์ยังไง?” หลายคนสงสัยว่า ถ้า Y มีแค่ “มีโรค” (1) หรือ “ไม่มีโรค” (0)...

Four Essential Assumptions for Causal Inference: Exchangeability, Positivity, No Interference, Consistency, and SUTVA
1. Exchangeability (Ignorability) 🔎 What it means: After adjusting for the observed covariates X, the treated and untreated groups are...

Causal Inference in Observational Research: Strategies, Assumptions, and Stata Tools
1 Why Observational Data Are Harder Than RCTs RCT privilege What you lose in an observational study Investigator controls treatment →...

Causal Inference in RCTs: ITT, CACE, and Estimands in Practice
Causal Inference inside an RCT – STATA-First Edition 0 Glossary of Key Abbreviations Short-form Stands for Meaning in this document R...